import numpy as np
from IPython.display import Image, display
# Import local plotting functions and in-notebook display functions
import matplotlib.pyplot as plt
%matplotlib inline
import sys
sys.path.append('../') # Add the course directory to the Python path
import cphy.plotting as cplot
Microscopy data and deconvolution methods¶
Many microscopy techniques are based on the detection of light emitted by fluorescent molecules. The light emitted by a single molecule is very weak and cannot be detected directly. Instead, the sample is illuminated with a strong light source, and the light emitted by the molecules is detected by a camera.
The image obtained in this way is a convolution of the true image with the point spread function (PSF) of the microscope. The PSF is the image of a single molecule under the microscope, and it can often be calculated experimentally or inferred from the optical design imaging setup. Mathematically, the observed image has the form of a convolution between the true image and the PSF:
$$ \begin{align} I_{obs}(x,y) &= (I_{true} * PSF)(x,y) \\ &= \int_{-\infty}^{\infty} \int_{-\infty}^{\infty} I_{true}(x',y') PSF(x-x',y-y') dx' dy' \end{align} $$
Note the similarity between this equation and the Green's function of a physical system.
In order to increase the effective resolution of the microscope, it is necessary to deconvolve the image with the PSF. This is an ill-posed problem, because there are many different methods for solving it.
fpath = "../resources/fluorescence_image.npy"
im = np.load(fpath, allow_pickle=True)
plt.imshow(im)
cplot.vanish_axes()
## Deconvolve the image
import scipy.fftpack# import fft2, ifft2, fftshift, ifftshift
from scipy.signal import convolve2d
def gaussian_kernel(size: int, sigma: float):
kernel = np.fromfunction(
lambda x, y: (1/ (2 * np.pi * sigma**2)) *
np.exp(- ((x - (size - 1) / 2) ** 2 + (y - (size - 1) / 2) ** 2) / (2 * sigma**2)),
(size, size)
)
return kernel / np.sum(kernel)
def richardson_lucy(image, sigma=1, iterations=50):
image = image.astype(np.float64) #/ np.max(image)
psf = gaussian_kernel(3, sigma)
estimate = np.copy(image)
for i in range(iterations):
q = image / (1e-8 +convolve2d(estimate, psf, 'same'))
estimate *= convolve2d(q, psf[::-1, ::-1], 'same')
return estimate.T
all_channels = []
for i in range(3):
channel = richardson_lucy(im[..., i])
channel = 255 * channel / np.max(channel)
all_channels.append(channel)
restored_image = np.array(all_channels).T
restored_image = restored_image.astype(np.uint8)
# Show image a and restored side-by-side
plt.figure(figsize=(10, 5))
plt.subplot(121)
plt.imshow(im)
plt.title("Original")
cplot.vanish_axes()
plt.subplot(122)
plt.imshow(restored_image)
plt.title("Deconvolved")
cplot.vanish_axes()
# Compare just green channel to highlight what changed
plt.figure(figsize=(10, 5))
plt.subplot(121)
plt.imshow(im[..., 1])
plt.title("Original")
plt.subplot(122)
plt.imshow(restored_image[..., 1])
plt.title("Deconvolved")
Text(0.5, 1.0, 'Deconvolved')
Superresolution: inferring latent structure from low-resolution observations¶
Models with latent variables¶
- Gaussian mixture models(unsupervised model)
- Clustering (unsupervised)
- Unsupervised learning: we want to learn the underlying structure of the data
Bayes' Theorem and Maximum-Likelihood¶
If $X$ denotes our observed data, and $\theta$ denotes the parameters of our model of the data's generating process, $$ p(X | \theta)\, p(\theta) = p(\theta |X)\, p(X) $$ The individual terms in Bayes' theorem have distinct interpretations.
- The distribution over model parameters, $p(\theta)$, represents the prior; in the absence of any observed data, the prior tells us our expectation regarding the model values. This distribution might incorporate domain knowledge or previous fitted models.
- The distribution $p(X)$ represents the distribution of observable data, which depends on factors external to the model.
- The conditional probability $p(\theta | X)$ represents the posterior of the model: given our observations, $X$, the posterior determines the probability of different models.
- The conditional probability $p(X | \theta)$ represents the likelihood, which is the probability of having observed a given set of observations $X$ given a specific model with parameters $\theta$.
Many classes of algorithms attempt to determine $\theta$ through maximumum likelihood methods, which attempt to find the model $\theta$ that maximizes the probability of the observed dats $X$.
Superresolution: localizing emitters in blurry images¶
Superresolution microscopy aims to image systems in visible light below the classical diffraction limit. This can be useful for studying chemical and biological systems (which are often probed using fluorophores that emit visible fluorescence), but similar methods have been adapted for other wavelengths and imaging modalities.
In superresolution microscopy, we attempt to beat the diffraction limit by localizing the emitter of individual photons.
For example, if the point spread function of a point source is a Gaussian, we want to localize the emitter to the mean of the Gaussian.
In some advanced superresolution methods, like PALM/STORM, only a subset of all fluorescent sources are activated at a given time, thus reducing the likelihood of two sources overlapping and thereby improving centroid localization
Here, we are going to work with a fluorescence microscopy image where we assume that we have a gaussian point spread function. We are going to fit the image to a mixture of Gaussians, in order to gain information about the subpixel features.
Pores in a cell's nuclear envelope¶
This image shows the edge of a cell's nuclear envelope, where genetic material (chromatin) has been stained in blue, nuclear pores in red, and the envelope itself in green. The upper panels show a typical florescence microscopy dataset, while the lower panels show higher-resolution images taken using more advanced imaging techniques
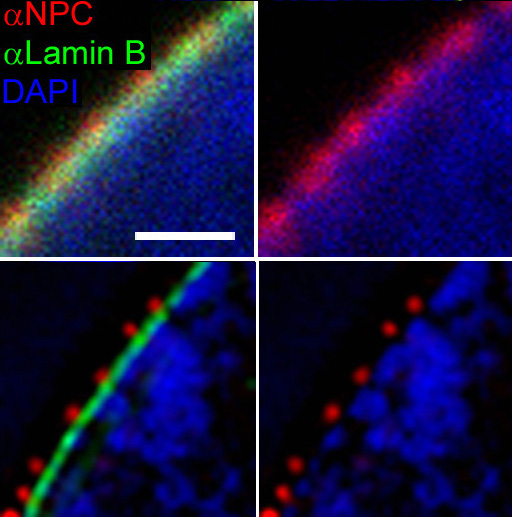
Given data from the upper panel, can we infer the locations of the nuclear pores?¶
- We are going to treat each red-channel pixel as a photon emitter, and we can simulate a set of photon detections from the emitter
from PIL import Image
im_path = "../resources/wikimedia_3D-SIM-1_NPC_Confocal_vs_3D-SIM_detail.jpg"
# Open the image form working directory
im = Image.open(im_path)
## Let's excerpt the red channel
im_excerpt = np.array(im)[:210, -225:-20, 0]
# plt.figure()
# plt.imshow(im_excerpt, extent=[0, 1, 0, 1], origin='lower')
## Sample data from the image---photons, counts, etc.
im_excerpt = im_excerpt / np.sum(im_excerpt)
im_excerpt = im_excerpt ** 2
im_excerpt = im_excerpt / np.sum(im_excerpt)
np.random.seed(0)
index = np.random.choice(np.prod(im_excerpt.shape), size=500, p=im_excerpt.ravel())
X = np.array(np.unravel_index(index, shape=im_excerpt.shape)).T.astype(float)
X /= X.max(axis=0) # scale to unit square
plt.plot(X[:, 0], X[:, 1], '.r')
plt.title("Photons detected in the red channel")
plt.axis('off')
# ## Let's try to fit a Gaussian mixture model to this data
# from sklearn.mixture import GaussianMixture
# gmm = GaussianMixture(n_components=4, covariance_type='full')
# gmm.fit(X)
# ## Let's plot the results
# plt.plot(gmm.means_[:, 0], gmm.means_[:, 1], '*w', markersize=20)
(0.005263157894736831, 1.0473684210526315, -0.05, 1.05)
Gaussian mixture models as probabilistic machine learning¶
Gaussian mixture models are a type of generative model that assume that the data is generated by a mixture of $K$ Gaussian distributions.
The trainable parameters of the model are the means, covariances, and relative weights of the Gaussians. We can say that the observed data is conditioned on the trainable parameters of the model.
The main hyperparameter of the model is the number of Gaussians, $K$.
We see each observed datapoint $X_i$ as being generated by one of the $K$ Gaussians, with probability $p_i$ of being generated by the $i$th Gaussian.
$$ p(X_i | \boldsymbol{\theta}) = \sum_{k=1}^K w_i \mathcal{N}(X_i | \mu_k, \Sigma_k) $$ where $\boldsymbol{\theta} = \{w_k, \mu_k, \Sigma_k\}_{k=1}^K$ are the parameters of the model.
The learning problem therefore consists of finding the parameters $\mu_k, \Sigma_k, w_k$ that maximize the likelihood of the observed data. The likelihood of the data is given by the product of the likelihoods of each datapoint:
$$ p(X | \boldsymbol{\theta}) = \prod_{i=1}^N p(X_i | \boldsymbol{\theta}) $$
The log-likelihood of the observed data is therefore given by
$$ \log p(X | \boldsymbol{\theta}) = \sum_{i=1}^N \log p(X_i | \boldsymbol{\theta}) $$
How do we fit a mixture of Gaussians to a dataset?¶
EM algorithm¶
We recently clustered data using the k-means algorithm. The k-means algorithm is a special case of the expectation-maximization (EM) algorithm. Recall that the k-means algorithm works by iteratively assigning data points to clusters and then updating the cluster centers. The EM algorithm is a more general method for learning models with latent variables.
For learning generative models with latent variables in cases where we don't have gradient information to perform traditional optimization. For example, we have data that we suspect has been sampled from a family of probability distributions. The sampling itself is a non-invertible and non-differentiable process
Common ML methods that use the EM algorithm are: hidden Markov models, clustering, and mixture models
Broadly, the EM algorithm has two steps:
- Expectation. We create an initial estimate for the distribution parameters $\theta$, and we then calculate the posterior probability different datapoints under the initial model, $p(X | \theta)$. These values are sometimes called the "responsibilities."
- Maximization. We update our estimate of $\theta$ in order to maximize the likelihood of the training dataset.
For more discussion of the EM algorithm, Gaussian mixtures, and clustering, see section 2.1 of this paper
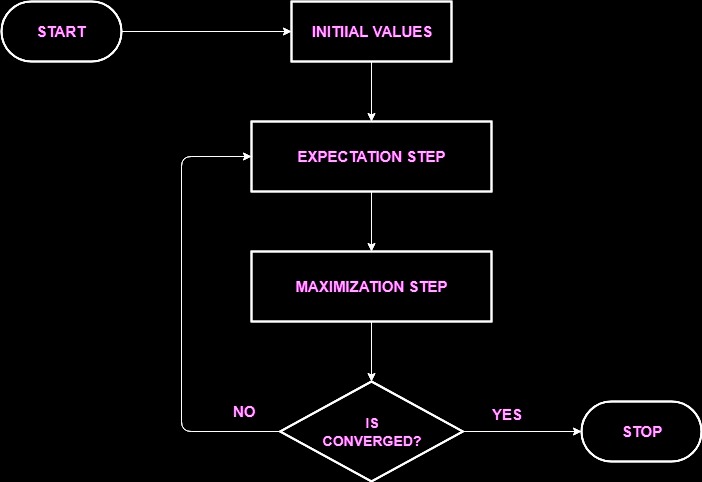
Image from source
The EM algorithm for Gaussian mixtures¶
Expectation step¶
In the expectation step, we calculate the posterior probability of each datapoint $X_i$ being generated by each of the $K$ Gaussians at their current positions. This is called the "responsibility" of the $k$th Gaussian for the $i$th datapoint:
$$ \gamma_{ik} = \frac{w_k \mathcal{N}(X_i | \mu_k, \Sigma_k)}{\sum_{j=1}^K w_j \mathcal{N}(X_i | \mu_j, \Sigma_j)} $$
We can think of the responsibilities as the probability that the $i$th datapoint was generated by the $k$th Gaussian.
Maximization step¶
In the maximization step, we use the responsibilities to update the parameters of the Gaussians to maximize the likelihood of the data. The update equations are given by
$$ \begin{align} \mu_k &= \frac{1}{N_k} \sum_{i=1}^N \gamma_{ik} X_i \\ \Sigma_k &= \frac{1}{N_k} \sum_{i=1}^N \gamma_{ik} (X_i - \mu_k)(X_i - \mu_k)^T \\ w_k &= \frac{N_k}{N} \end{align} $$
where $N_k = \sum_{i=1}^N \gamma_{ik}$ is the total responsibility of the $k$th Gaussian.
from scipy.stats import multivariate_normal
class GaussianMixtureModel:
"""
An implementation of the EM algorithm for Gaussian mixture models.
Attributes:
n_components (int): The number of mixture components.
n_iter (int): The number of EM iterations to perform.
tol (float): The convergence threshold. EM iterations will stop when the
log likelihood average gain is below this threshold.
store_history (bool): Whether or not to store the history of the means
at each step.
learning_rate (float): The learning rate for the gradient descent update.
random_state (int): The random seed.
"""
def __init__(self, n_components, n_iter=100, tol=1e-6, store_history=False,
learning_rate=1.0, random_state=None
):
self.n_components = n_components
self.n_iter = n_iter
self.tol = tol
self.learning_rate = learning_rate
self.random_state = random_state
self.store_history = store_history
if self.store_history:
self.history = list()
## This special method determines the output of print(GaussianMixtureModel())
def __str__(self):
return "GaussianMixtureModel(n_components={})".format(self.n_components)
def initialize(self, X):
"""Initialize the GMM parameters.
Args:
X (array-like): Training data with shape (n_samples, n_features)
Returns:
self
"""
n_samples, n_features = X.shape
np.random.seed(self.random_state)
# Initialize the weights to equal
self.weights = np.ones(self.n_components) / self.n_components
# Initialize the means randomly from the data
self.means = np.random.choice(X.flatten(), size=(self.n_components, n_features))
#self.means = np.random.random(size=(self.n_components, n_features)) * np.ptp(X, axis=0)
# Initialize the covariances
self.covariances = np.zeros((self.n_components, n_features, n_features))
for k in range(self.n_components):
self.covariances[k] = np.diag(np.random.rand(n_features))
return self
def pdf(self, X):
"""Compute the probability density function for each sample in X.
Args:
X (array-like): Training data with shape (n_samples, n_features)
Returns:
pdf (array-like): Probability density function for each sample in X.
"""
n_samples, n_features = X.shape
pdf = np.zeros((n_samples, self.n_components))
for k in range(self.n_components):
pdf[:, k] = self.weights[k] * multivariate_normal.pdf(X, self.means[k], self.covariances[k])
return pdf
def expectation(self, X):
"""Perform the expectation step.
Args:
X (array-like): Training data with shape (n_samples, n_features)
Returns:
responsibilities (array-like): The responsibilities for each sample in X.
"""
pdf = self.pdf(X)
responsibilities = pdf / pdf.sum(axis=1, keepdims=True)
return responsibilities
def maximization(self, X, responsibilities):
"""Perform the maximization step.
Args:
X (array-like): Training data with shape (n_samples, n_features)
responsibilities (array-like): The responsibilities for each sample in X.
Returns:
self
"""
n_samples, n_features = X.shape
# Update the weights
self.weights = responsibilities.sum(axis=0) / n_samples
# Update the means
self.means = (1 - self.learning_rate) * self.means + self.learning_rate * np.dot(responsibilities.T, X) / responsibilities.sum(axis=0, keepdims=True).T
#self.means_new = (1 - beta) * self.means_old + beta * self.means_new
# Update the covariances
for k in range(self.n_components):
diff = X - self.means[k]
self.covariances[k] = (1 - self.learning_rate) * self.covariances[k] + self.learning_rate * np.dot(responsibilities[:, k] * diff.T, diff) / responsibilities[:, k].sum()
return self
def fit(self, X):
"""Fit the model to the data X.
Args:
X (array-like): Training data with shape (n_samples, n_features)
Returns:
self
"""
self.initialize(X)
for i in range(self.n_iter):
# Expectation step
responsibilities = self.expectation(X)
# Maximization step
self.maximization(X, responsibilities)
# store the history
if self.store_history:
self.history.append(self.means.copy())
return self
def predict(self, X):
"""Predict the labels for X.
Args:
X (array-like): Training data with shape (n_samples, n_features)
Returns:
labels (array-like): The predicted labels for each sample in X.
"""
return self.expectation(X).argmax(axis=1)
gmm = GaussianMixtureModel(n_components=5, n_iter=2000, learning_rate=0.5, store_history=True, random_state=0)
gmm.fit(X)
labels = gmm.predict(X)
plt.imshow(im_excerpt, extent=[0, 1, 0, 1], origin='lower')
cmap = plt.cm.get_cmap('Spectral')
clrs = cmap(labels / np.max(labels))
plt.scatter(X[:, 0], X[:, 1], c=clrs, s=10)
# Plot centroids
plt.plot(gmm.means[:, 0], gmm.means[:, 1], '*w', markersize=20)
plt.axis('off')
/var/folders/xt/9wdl4pmx26gf_qytq8_d528c0000gq/T/ipykernel_73139/3659595493.py:6: MatplotlibDeprecationWarning: The get_cmap function was deprecated in Matplotlib 3.7 and will be removed two minor releases later. Use ``matplotlib.colormaps[name]`` or ``matplotlib.colormaps.get_cmap(obj)`` instead.
cmap = plt.cm.get_cmap('Spectral')
(0.0, 1.0, 0.0, 1.0)
## Make an interactive video
from ipywidgets import interact, interactive, fixed, interact_manual, Layout
import ipywidgets as widgets
def plotter(i):
fig = plt.figure(figsize=(10, 10))
plt.imshow(im_excerpt, extent=[0, 1, 0, 1], origin='lower')
plt.plot(gmm.history[i][:, 0], gmm.history[i][:, 1], '*w', markersize=20)
plt.xlim([0, 1])
plt.ylim([0, 1])
plt.show()
interact(
plotter,
i=widgets.IntSlider(0, 0, len(gmm.history) - 1, 1, layout=Layout(width='800px'))
)
interactive(children=(IntSlider(value=0, description='i', layout=Layout(width='800px'), max=1999), Output()), …
<function __main__.plotter(i)>
Super-resolution microscopy: PALM and STORM¶
Several superresolution methods localize the fluorescent emitter by exploiting temporal information---for example, if each emission site only intermittently releases photons, we can isolate overlapping emitters based on the timing of the photons.
Stages of a PALM/STORM experiment¶
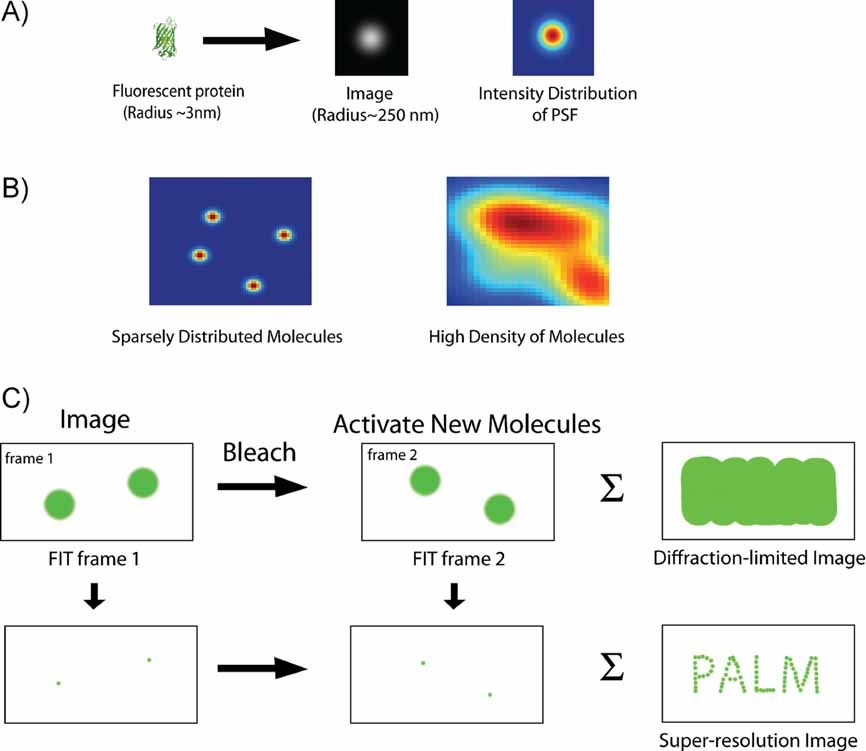
Stochastic Activation: A sparse subset of fluorophores (fluorescent molecules) is activated to a fluorescent state, ensuring that the activated fluorophores are well-separated and do not overlap within the diffraction-limited area.
Localization: The point spread function (PSF) of each activated fluorophore is acquired, often appearing as a diffraction-limited spot. A mathematical fit, commonly a 2D Gaussian, is used to find the center of the PSF, allowing the fluorophore's position to be determined with high precision.
Deactivation: After localization, the activated fluorophores are photobleached or switched back to a dark state, making them inactive.
Iteration: Steps 1-3 are repeated thousands to millions of times to build a dataset of highly precise fluorophore positions.
Reconstruction: The super-resolution image is reconstructed from the localized positions.
PALM typically uses photo-activatable or photo-switchable fluorescent proteins. Activation is generally irreversible, leading to photobleaching over long periods. In contrast, STORM employs synthetic dyes that can be cycled between dark and bright states multiple times, thus being more reusable compared to the fluorophores used in PALM.
These approaches can achieve resolutions on the order of 20-50 nm far beyond the visible diffraction limit (~200-300 nm). This technique was one of the ones honored by the 2014 Nobel Prize in Chemistry in 2014.
# show movie in notebook
fpath = "../resources/storm_superresolution.mp4"
from IPython.display import HTML
from base64 import b64encode
mp4 = open(fpath,'rb').read()
data_url = "data:video/mp4;base64," + b64encode(mp4).decode()
HTML("""
<video width=800 controls>
<source src="%s" type="video/mp4">
</video>
""" % data_url)
Glial cell microscopy data¶
To demonstrate EM methods for supperresolution data, we will examine a dataset of Glial cells from in the rat hippocampus. Glial cells are the "glue" of the nervous system---they provide structural support for neurons, and also provide nutrients and manage cerebrospinal fluid. The cells in the image have been stained with phalloidin, which causes actin within the cells to fluoresce. Because actin is often used in structural support, this allows us to see the shape of the cells. The cells were imaged using dSTORM
Our demonstration dataset will be a time series of images of glial cells. This dataset was produced and made available by Christophe Leterrier and Romain F. Laine, and the full dataset is available on Zenodo. The dataset is a downsampled version of the file BIN10_glia_actin_2D.tif by the original authors.
import matplotlib.animation
import IPython.display
# get the data
# im_subset = np.load("../resources/glial_cells.npy")
im_subset = np.load("../resources/glial_cells2.npy")
# def update(i):
# return im_subset[i]
# fig = plt.figure(figsize=(6, 6))
# IPython.display.HTML(matplotlib.animation.FuncAnimation(fig, update, frames=len(im_subset), interval=20).to_jshtml())
cplot.sliderplot(im_subset);
plt.show()
interactive(children=(IntSlider(value=0, description='i', layout=Layout(width='100%'), max=598), Output()), _d…
def gmm_mle_em_2d(data, n_components=1, max_iter=100, tol=1e-3, verbose=False):
"""
Perform subpixel localization using Gaussian Mixture Model with maximum likelihood
estimation and expectation maximization.
Args:
data (ndarray): the data to fit
n_components (int): the number of components to fit
max_iter (int): the maximum number of iterations
tol (float): the tolerance
verbose (bool): whether to print the log likelihood at each iteration
Returns:
centers (ndarray): the centers of the fitted components
covariances (ndarray): the covariances of the fitted components
weights (ndarray): the weights of the fitted components
"""
gmm = GaussianMixture(
n_components=n_components, max_iter=max_iter, tol=tol, verbose=verbose
)
gmm.fit(data)
centers = gmm.means_
covariances = gmm.covariances_
weights = gmm.weights_
return centers, covariances, weights
def gaussian_bump(X, width=1):
return np.exp(-np.sum((X / width) ** 2, axis=1))
def gaussian2d_mixture(centers, sigmas, size):
"""
Generate a 2D Gaussian mixture with given set of centers and sigmas on an array of
size `size`.
"""
# check if scalar
if np.isscalar(sigmas):
sigmas = sigmas * np.ones(centers.shape[0])
x = np.arange(size[0])
y = np.arange(size[1])
X, Y = np.meshgrid(x, y)
# convert X, Y to a list of points in 2d
X = np.array([Y.ravel(), X.ravel()]).T
out = gaussian_bump(X[..., None] - centers.T[None, :], width=sigmas)
return np.sum(out, axis=1).reshape(size)
# perform subpixel localization using Gaussian Mixture Model
# using maximum likelihood estimation and expectation maximization
import numpy as np
from sklearn.mixture import GaussianMixture
reconstruction_history = list()
upsample_factor = 2
reconstructed_image = np.zeros(upsample_factor * np.array(im_subset[0].shape))
for i, im in enumerate(im_subset):
if i % (im_subset.shape[0] // 25) == 0:
print(i, flush=True)
## Create candidate locations fluorophores based on local maxima
thresh = np.percentile(im, 80)
peaks = np.array(np.where(im > thresh)).T
## Refine and group peaks by fitting a Gaussian mixture model
peaks, covariances, weights = gmm_mle_em_2d(peaks, n_components=peaks.shape[0])
# reconstructed_image[peaks[:, 0], peaks[:, 1]] += 1
reconstructed_image += gaussian2d_mixture(
upsample_factor * peaks, 1, np.array(reconstructed_image.shape)
)
reconstruction_history.append(reconstructed_image.copy())
0 23 46 69 92 115 138 161 184 207 230 253 276 299 322 345 368 391 414 437 460 483 506 529 552 575 598
Examine our results¶
We compare the output to the simple mean of the image stack, which approximates what we would expect to see if we just took a long-exposure diffraction-limited image of the cell population. Technically our superresolution image upsampled the original image 2x; however, in order to faciliate comparison we downsample it back to the original resolution in order to emphasize new information gained by the superresolution algorithm.
minmaxscaler = lambda x : (x - np.min(x)) / (np.max(x) - np.min(x))
im_mean = np.mean(im_subset, axis=0)
# now make a single plot showing these side-by-side
plt.figure(figsize=(10, 5))
plt.subplot(121)
plt.imshow(minmaxscaler(im_mean), cmap='gray')
# hide all ticks
plt.xticks([]); plt.yticks([])
plt.title('Mean image')
plt.subplot(122)
plt.imshow(minmaxscaler(reconstructed_image)[::2, ::2], cmap='gray')
plt.xticks([]); plt.yticks([])
plt.title('Superresolution image')
Text(0.5, 1.0, 'Superresolution image')
# save the reconstruction history as a movie
frames = reconstruction_history
vmin, vmax = np.percentile(frames, 1), np.percentile(frames, 99)
for i in range(len(frames) - 1):
out_path = "../private_dump/superresolution/frame" + str(i).zfill(4) + ".png"
plt.figure()
plt.imshow(frames[i], cmap="viridis")
ax = plt.gca()
ax.set_axis_off()
ax.xaxis.set_major_locator(plt.NullLocator())
ax.yaxis.set_major_locator(plt.NullLocator())
ax.set_aspect(1, adjustable='box')
cplot.vanish_axes()
plt.savefig(out_path, bbox_inches='tight', pad_inches=0.0, dpi=300)
plt.close()
Questions¶
In traditional photography, there is a well-documented tradeoff between exposure time (total light collected) and temporal resolution. What experimental parameters would you adjust to trade off between these two quantities in a superresolution experiment?
Suppose that whether a given fluorophore is in the on or off state is a Poisson process with rate $\lambda$. What is the probability that a given fluorophore is in the on state at time $t$? Given $N$ underlying fluorophores with the same rate $\lambda$, what is the expected number and variance of fluorophores in the on state at a given time $t$?
What inductive biases are present in our use of a Gaussian mixture model to model the distribution of fluorophore positions? What are the advantages and disadvantages of these biases?